Mechanics of Materials
.A big part of your design, is a consideration of the material that you are using.
.
Mechanics of Materials is the study of the mechanical properties of ceramics, metals, and polymers, and the role of processing and microstructure in controlling these properties.
.
Common Material properties to consider:
Ductility, strength, hardness, thermal expansion, thermal conductivity, electrical conductivity, corrosion properties, melting point, density, etc.
.
.
.
Shear Modulus (or Modulus of Rigidity)
elasticity for a shearing force.
"the ratio of shear stress to the displacement per unit sample length (shear strain)"
.


Shear Modulus for common materials:
http://www.engineeringtoolbox.com/modulus-rigidity-d_946.html
Poisson’s ratio:
.


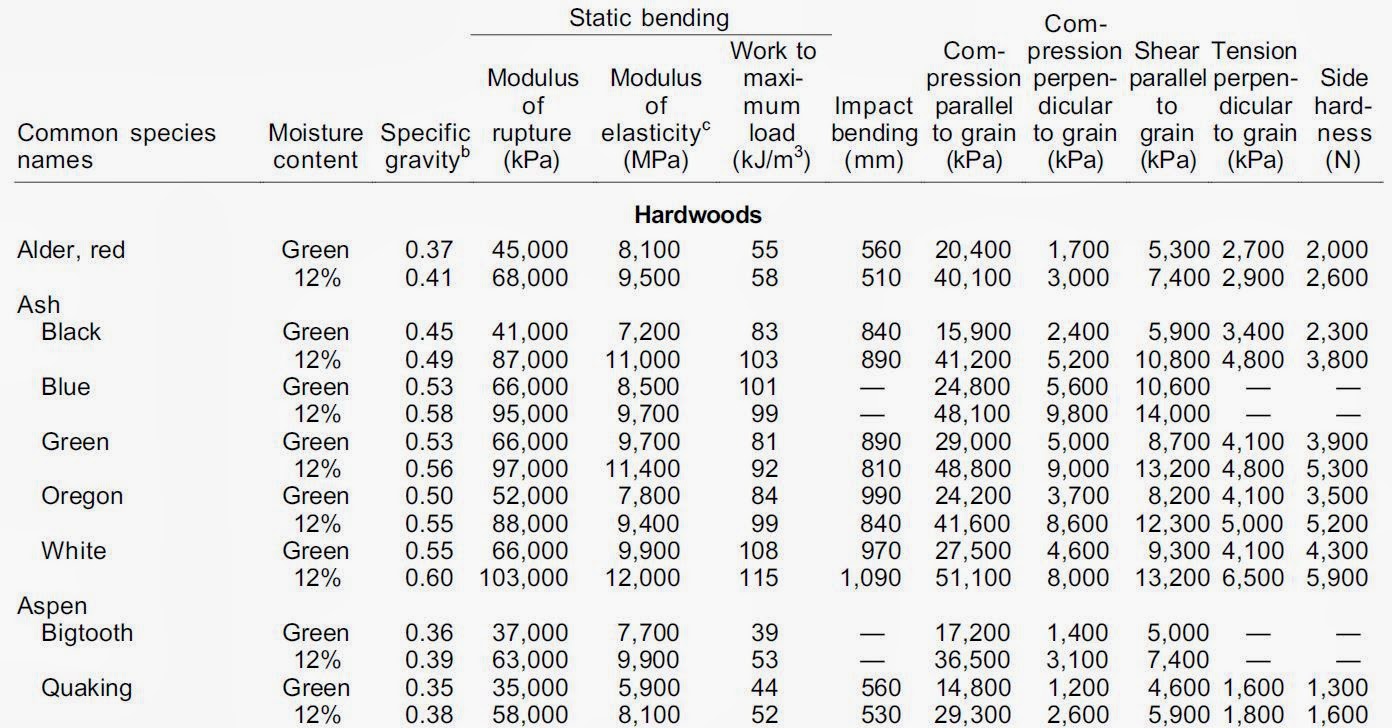
Mechanical Properties of Wood:.

http://www.conradlumberco.com/pdfs/ch4-Mechanical-Properties-of-Wood.pdf
Wood: natural, heterogeneous material with knots, splits, non-uniform grain, and variable properties.
Testing
Clear – no knots, uniform straight grain, no splits, homogeneous, defined moisture content - ideal case giving the largest values of forces. (Generally, knots etc. lower strength properties)
Lots of tests, get average values with large standard deviations.
Orthotropic Nature – Strength properties depend on orientation of grain to force.

http://www.conradlumberco.com/pdfs/ch4-Mechanical-Properties-of-Wood.pdf
Wood: natural, heterogeneous material with knots, splits, non-uniform grain, and variable properties.
Testing
Clear – no knots, uniform straight grain, no splits, homogeneous, defined moisture content - ideal case giving the largest values of forces. (Generally, knots etc. lower strength properties)
Lots of tests, get average values with large standard deviations.
Orthotropic Nature – Strength properties depend on orientation of grain to force.


Because wood is orthotropic, there are 12 different strength measurements:
3 different Young's modulus (one for each direction)
3 Shear modulus (G)
6 Poisson’s ratios
Example tables: http://www.conradlumberco.com/pdfs/ch4-Mechanical-Properties-of-Wood.pdf
Metals:
14 basic bravais-lattices structures of atoms:

Materials properties are controlled by how easily planes of atoms can slide over one another. Cubic lattice structures allow slippage to occur more easily than non-cubic lattices, and so are more ductile.
.Solidification:
Nucleation - when crystals (grains) start to grow

Crystal Packing defects change material properties
linear defects - which are groups of atoms in irregular positions. Linear defects are commonly called dislocations.
planar defects - which are interfaces between homogeneous regions of the material. Planar defects include grain boundaries, stacking faults and external surfaces.
Grain boundaries - stop slip planes, and strengthen materials.
The size of the grains depends on how fast you cool it, and how many nucleation sites you get.
Phase diagram of solidification:



Steel
.

Steel is an iron alloy, with up to 2.1% Carbon by weight.
Alloy - mixture of a metal + non-metalLow carbon steel (mild steel) contains less than 0.3% carbon.
Medium carbon steels contain carbon from 0.3 -0.55%.
High carbon steel contain more than 0.5% carbon.
Iron with more than 2% carbon is referred to as Cast Iron.
.
Effect of Carbon on steel:.
Increase in carbon in steel:
1) Decreases the ductility of steel.
2) Increases the tensile strength of steel
3) Increases the hardness of steel.
4) Decreases the ease with which steel can be machined.
5) Lowers the melting point of steel.
6) Makes steel easier to harden with heat treatments.
7) Lowers the temperature required to heat treat steel.
8) Increases the difficulty of welding steel.
.
Steel with 0.2% Carbon can attain Rockwell C hardness of 49, while an 0.8% carbon steel can be hardened to Rockwell C of 65.
As carbon is added, steel gets harder and becomes difficult to machine.
.
Example: steels for springs must have at least 0.45 % carbon to attain required hardness.
Carbon steels, and alloy steels are designated by a four digit number:
- first digit indicates = the main alloying element(s),
- second digit = the secondary alloying element(s),
- last two digits = the amount of carbon,
Example
1060 steel = a plain-carbon steel containing 0.60 wt% C
Carbon Steels
Carbon
steels contain trace amounts of alloying elements and account for
90% of total steel production. Carbon steels can be further categorized
into three groups depending on their carbon content:
- Low Carbon Steels/Mild Steels contain up to 0.3% carbon
- Medium Carbon Steels contain 0.3 – 0.6% carbon
- High Carbon Steels contain more than 0.6% carbon
Alloy Steels
Alloy steels contain alloying elements
(e.g. manganese, silicon, nickel, titanium, copper, chromium, and aluminum) in varying proportions in order to
manipulate the steel's properties, such as its hardenability, corrosion resistance, strength, formability, weldability or
ductility.
Applications
for alloys steel include pipelines, auto parts, transformers, power generators
and electric motors.
Stainless Steels
Stainless
steels generally contain between 10-20% chromium as the main
alloying element and are valued for high corrosion resistance. With over 11%
chromium, steel is about 200 times more resistant to corrosion than mild steel.
These steels can be divided into three groups based on their crystalline
structure:
- Austenitic: Austenitic steels are non-magnetic and non
heat-treatable, and generally contain 18% chromium, 8% nickel and less
than 0.8% carbon. Austenitic steels form the largest
portion of the global stainless steel market and are often used in food
processing equipment, kitchen utensils, and piping.
- Ferritic: Ferritic steels contain trace amounts of nickel, 12-17% chromium, less than 0.1% carbon, along with other alloying elements, such as molybdenum, aluminum or titanium. These magnetic steels cannot be hardened by heat treatment but can be strengthened by cold working.
- Martensitic: Martensitic steels contain 11-17% chromium, less
than 0.4% nickel, and up to 1.2% carbon. These magnetic and heat-treatable
steels are used in knives, cutting tools, as well as dental and surgical
equipment.
Tool Steels
Tool
steels contain tungsten, molybdenum, cobalt and vanadium in varying quantities
to increase heat resistance and durability, making them ideal for cutting and
drilling equipment.
Steel products can also be divided by
their shapes and related applications:
- Long/Tubular Products include bars and rods, rails, wires, angles,
pipes, and shapes and sections. These products are commonly used in the
automotive and construction sectors.
- Flat Products include plates, sheets, coils, and strips. These
materials are mainly used in automotive parts, appliances, packaging,
shipbuilding, and construction.
- Other Products include valves, fittings, and flanges and are
mainly used as piping materials.
Polymers - an organic material created with a chain of carbon atoms. Includes rubber and synthetic materials such as plastics and elastomers. Can have a wide range of mechanical properties and colors.
Made up of chains:
The repeating unit in a polymer chain
Monomer –
A single mer unit (n=1)
Polymer –
Many mer-units along a chain (n=103 or more)
Degree of Polymerization –
The average number of mer-units in a chain.
Applied Stress - chains stretch out.
Length of the polymer chain:
0-100 atoms = liquid
100 + atoms = waxy solid
1,000 + = solid (polyethylene etc.) with definable material properties of strength, ductility, hardness, etc.
increased length = increased binding force between molecules.
Chains are a tangled mess (picture a mass of intertwined worms randomly thrown into a pail)
Ceramics:
an inorganic, nonmetallic solid that is prepared from powdered materials and is fabricated into products through the application of heat
Ceramics generally have strong covalent and ionic bonding which produce:
- high hardness,
- high compressive strength,
- low ductility
- low tensile strength
- chemically inert
- Poor electrical and thermal conductors.
Atomic microstructures widely vary from simple to complex.
- Glass - amorphous
- Crystalline
- Crystalline + glassy






































